19+ Letter To File Fda
On October 25 2017 FDAs Center for Devices and Radiological Health CDRH issued two final guidance documents that provide FDAs. Freedom of Information Act FOIA Call 301-796-3900.

The Safety And Efficacy Of 3 4 Methylenedioxymethamphetamine Assisted Psychotherapy In Subjects With Chronic Treatment Resistant Posttraumatic Stress Disorder The First Randomized Controlled Pilot Study Michael C Mithoefer Mark T Wagner Ann T
Web When it comes to changes to a medical device here are the regulatory options.
. However we are unable to grant final approval with respect. FDAs FOI Points of Contact. Food and Drug Administration 10903 New Hampshire Ave Silver Spring MD 20993.
Documented per QSR design change control requirements no letter to. Web As soon as possible but no later than 14 days from the date of this letter submit the content of labeling 21 CFR 31450l in structured product labeling SPL format using the FDA. FDA-2021-D-0861 Cover Letter Attachments for Controlled Correspondence and Abbreviated New Drug Application Submissions.
Food and Drug Administration FDA have sent warning letters to companies allegedly selling unapproved products that may violate. Hannah Beier Bloomberg via Getty. Web 67 rows Letters to Industry.
Find an FDA staff member. Web A health care worker administers a dose of the Novavax Covid-19 vaccine at a pharmacy in Schwenksville Pa on Aug. 1 the reasons why the FDA refused to file applications and.
Web Main Outcomes and Measures Two types of information were extracted and cataloged from RTF letters. Web February 19 2020 The Honorable Dr. Web that was used to check the files for viruses.
Web The drug overdose and addiction crisis collided with the COVID-19 pandemic with the potential to worsen the negative impacts of each for individuals. Web Print Mail Download i. 314101 Filing an NDA and receiving an ANDA.
Information on submitting SPL files using eLIST may be found in the guidance for industry SPL Standard for Content of Labeling Technical Qs. Web The collections of information for Form FDA 356h NDA and ANDA cover letter have been approved under OMB control number 0910-0338. Web View the list Emergency Use Authorizations for Drugs and Non-Vaccine Biological Products for additional information including letters of authorization fact.
The FDA has expanded its list of eye drops recalled in 2023 because the products could. Web This letter is in reference to your abbreviated new drug application ANDA received for. Cover Letter Components.
Matters described in FDA warning letters may have been subject to subsequent interaction between FDA. Web Contact FDAs Office of Media Affairs. Web Food and Drug Administration Docket No.
Web FDA warns against using 26 eye drop products due to infection risk 0033. This webpage is a resource for external stakeholders that contain correspondence issued by the Center for Devices and. Web August 21 2021.
Effective February 19 2021. A Filing an NDA. Applicant should use cover letters to help the FDA identify the content and.
Web 11 rows Learn about the types of warning letters on FDAs website. Hahn Commissioner of Food and Drugs US. 1 Within 60 days after FDA receives an NDA the Agency will determine whether the NDA.
Web Your letter attempted to shift the blame from drug manufacturers who have a long history of using their monopoly power to raise prices to other actors in the drug. Web This article provides a comprehensive overview of what a Letter to File is the purpose of writing one and the appropriate situations in which a company might use. Web The Federal Trade Commission and US.

Fda Rejection Of Acorda S Inbrija Is Another Challenge After Ampyra Patent Setback Pharmaceutical Technology

Fda Warning Letter To Neil R Feins M D 2009 05 20 Circare

Help Pubmed

Fda Responds To Our Concerns About Their Temporary Policy On Food Labeling Changes Gluten Free Watchdog
Coronavirus Covid 19 And Medical Devices Fda

Fake Fda Warning Letter Scam The Niche

Fda Investigating Reports Of Hospitalizations After Fake Ozempic Wltx Com
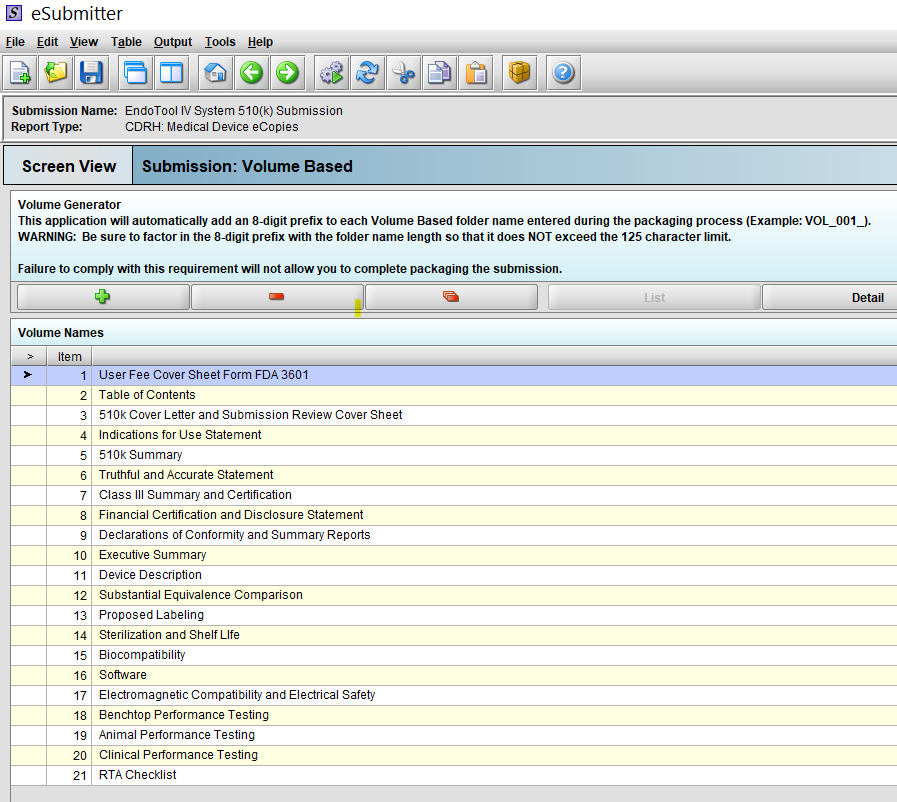
What Is 510k Content Format Medical Device Academy
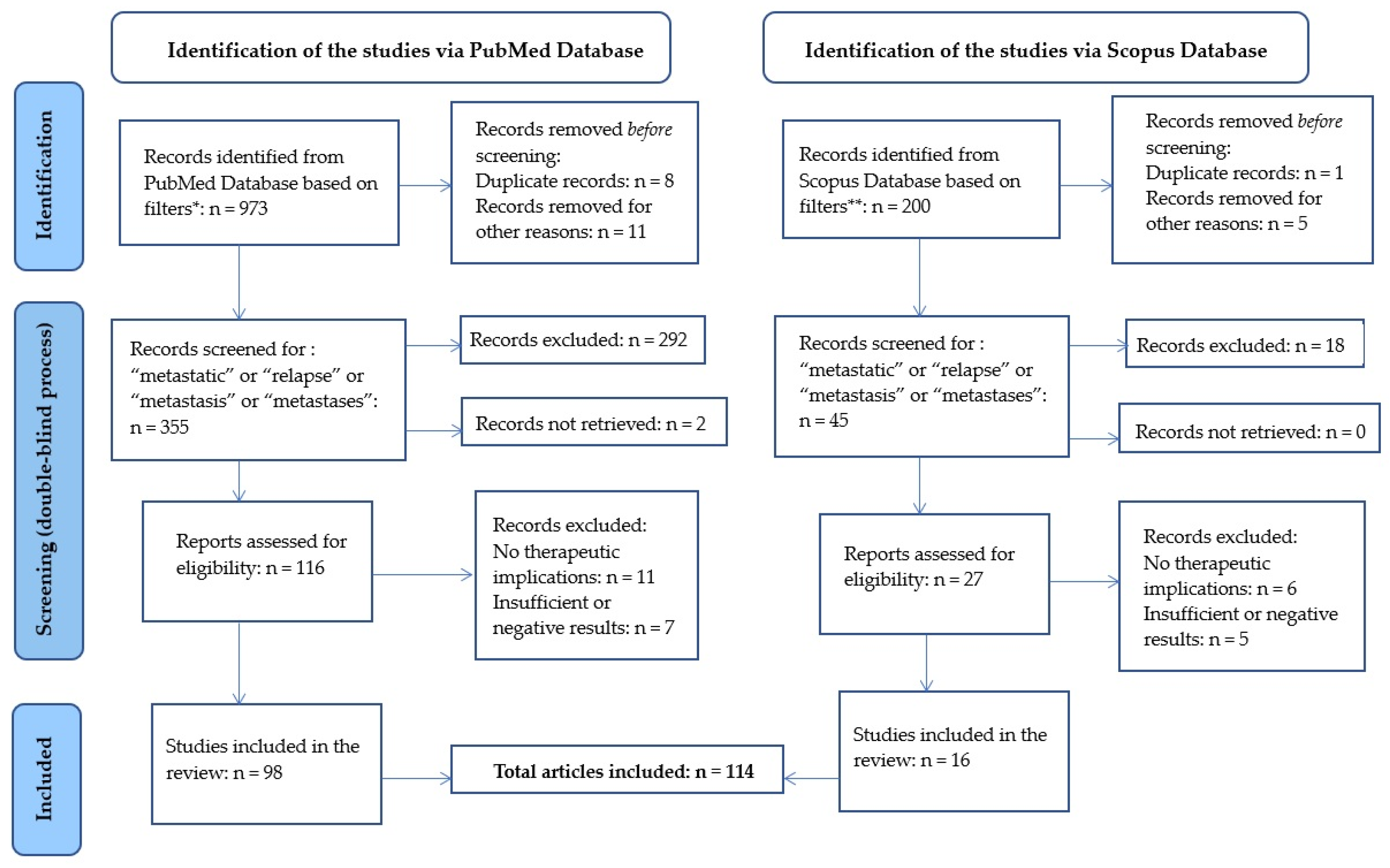
Biomedicines Free Full Text Update On Classic And Novel Approaches In Metastatic Triple Negative Breast Cancer Treatment A Comprehensive Review

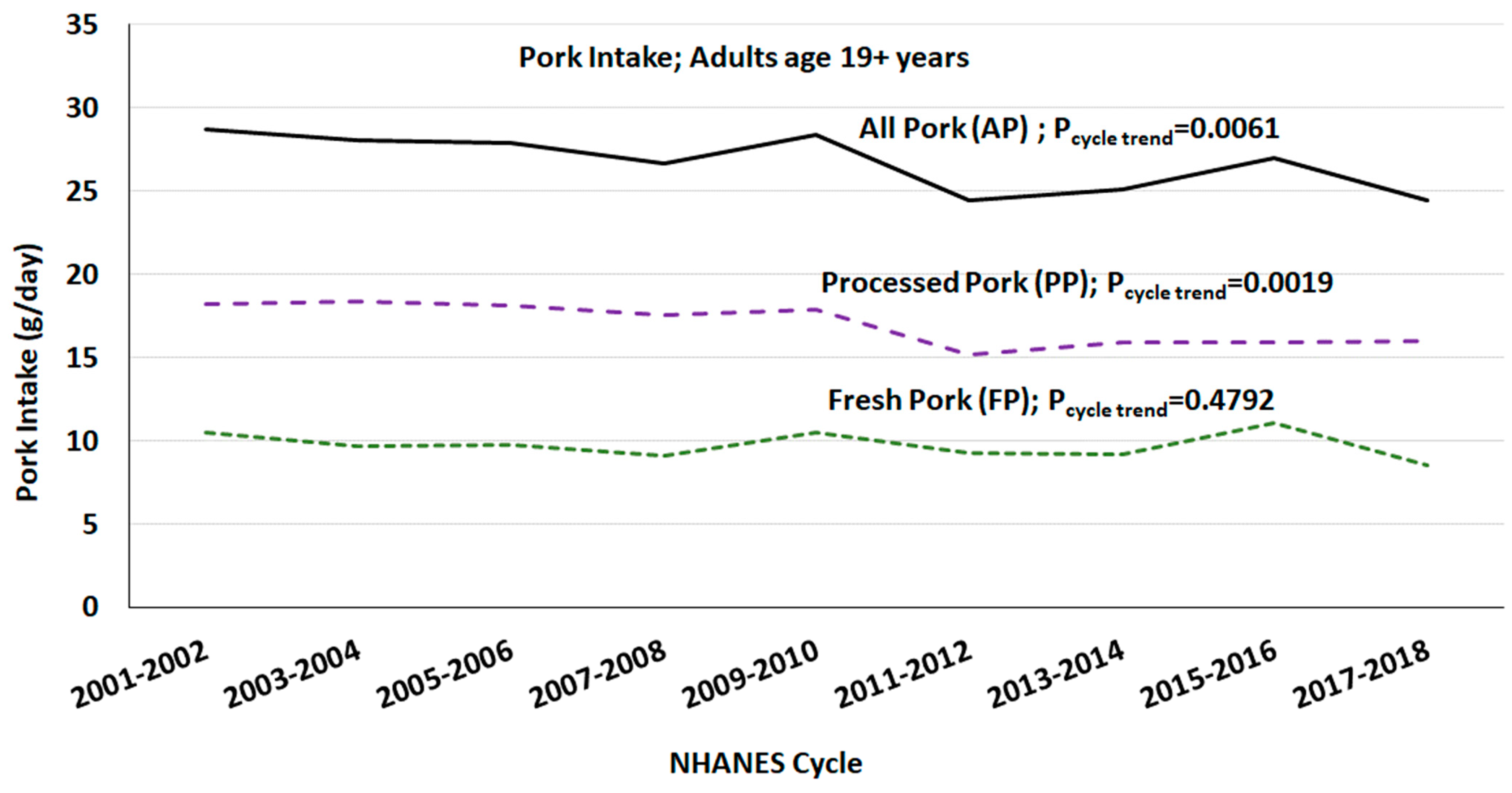
Tree Nut Snack Consumption Is Associated With Better Diet Quality And Cvd Risk In The Uk Adult Population National Diet And Nutrition Survey Ndns 2008 2014 Public Health Nutrition Cambridge Core
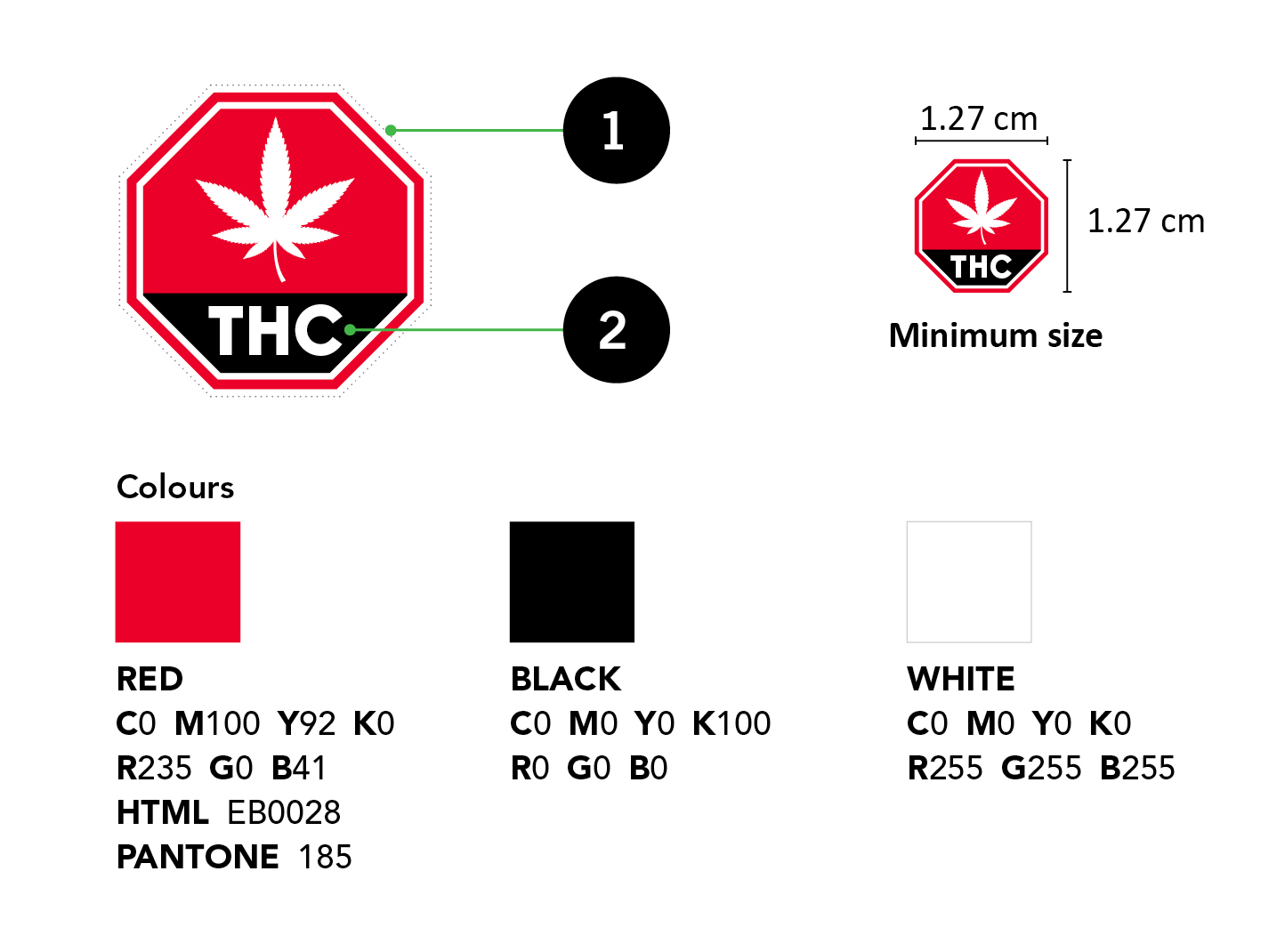
Packaging And Labelling Guide For Cannabis Products Canada Ca

Fda Warning Letter Ipq

Frontiers Effectiveness Of Telerehabilitation On Motor Impairments Non Motor Symptoms And Compliance In Patients With Parkinson S Disease A Systematic Review

The Safety And Efficacy Of 3 4 Methylenedioxymethamphetamine Assisted Psychotherapy In Subjects With Chronic Treatment Resistant Posttraumatic Stress Disorder The First Randomized Controlled Pilot Study Michael C Mithoefer Mark T Wagner Ann T

2019 Acc Aha Guideline On The Primary Prevention Of Cardiovascular Disease Executive Summary A Report Of The American College Of Cardiology American Heart Association Task Force On Clinical Practice Guidelines Circulation

Policy Research For Front Of Package Nutrition Labeling Developing And Testing A Summary System Algorithm Aspe

Immunizations Billing Manual Colorado Department Of Health Care Policy Financing